This page was last updated on May 15th, 2019 at 08:10 pm
Use case 1: docking box define using a known ligand
In this scenario we have a receptor and a known ligand in the PDBQT format and we define a docking box centered on the ligand.
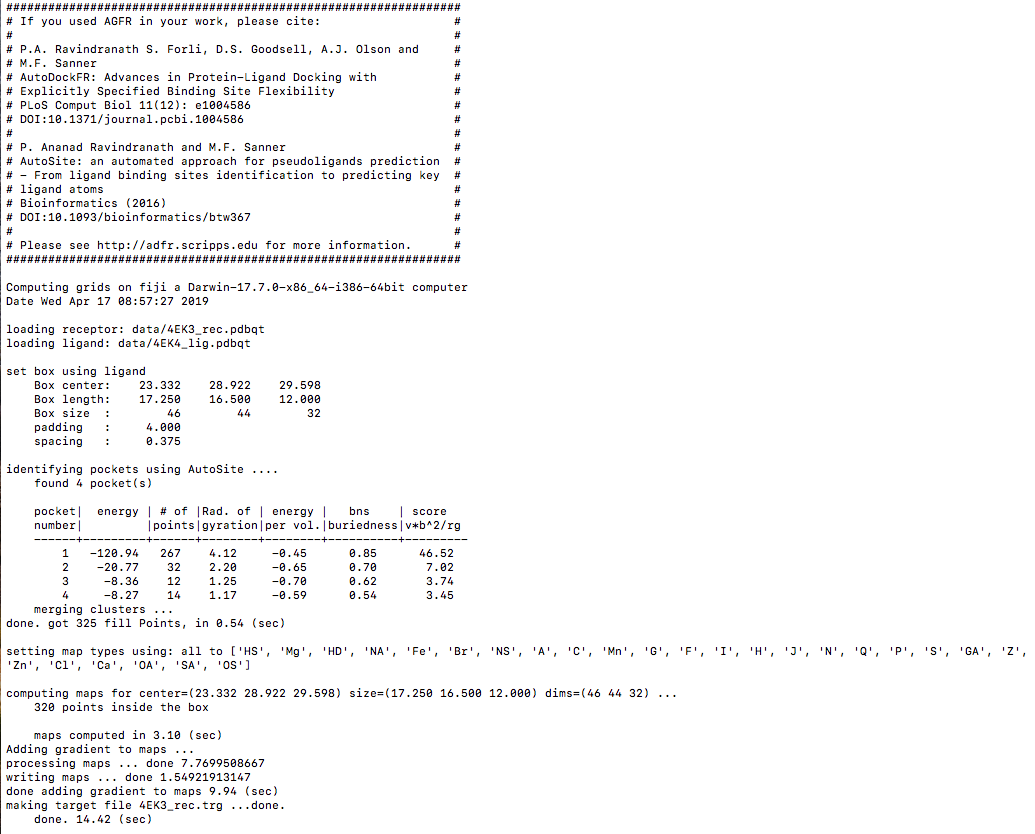
We will use the cyclic dependent kinase protein 2 CDK2 (pdbid:4EK3) and one of its ligands (pdbid:4EK4). The files 4EK3_rec.pdbqt and 4EK4_lig.pdbqt are available in the data file associated with this tutorial.
run ADFRsuite-1.0/bin/agfrgui to start the Graphical User interface for these tutorials.
Click  and select 4EK3_rec.pdbqt
and select 4EK3_rec.pdbqt
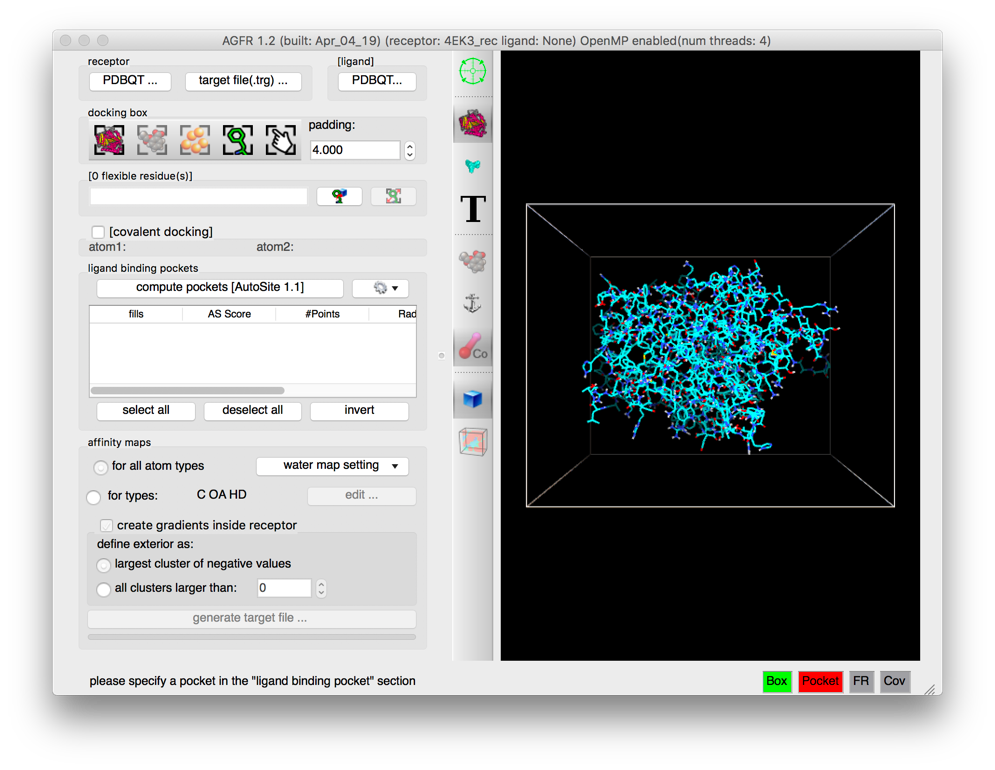
Details: the receptor molecule is loaded and displayed as line representing atomic bonds colored by atom type with carbon atoms shown in cyan. The default docking-box covers the entire receptor with the default padding (4.0 Angstroms) added to each side.
NOTES:
- Amino acids located in the docking box with no flexible side-chains (i.e. glycine, alanine and proline) are displayed with dimmed down lines.
- Several buttons in the control section and the tool-bar are now enabled.
- The status bar indicates that the next step could be to detect pockets.
The 3D scene can be rotated, translated and scaled using the 3 mouse buttons:
| Mouse button | Action |
| Left | Rotate |
| Middle | Translate |
| Right | Scale |
Depth-cueing can be turned on and off by pressing the keyboard key ‘d’ while the mouse pointer is in the 3D view.
Click on the  button to load 4EK4_lig.pdbqt
button to load 4EK4_lig.pdbqt
Details: the ligand molecule appears in the licorice representation with carbon atoms displayed in yellow.
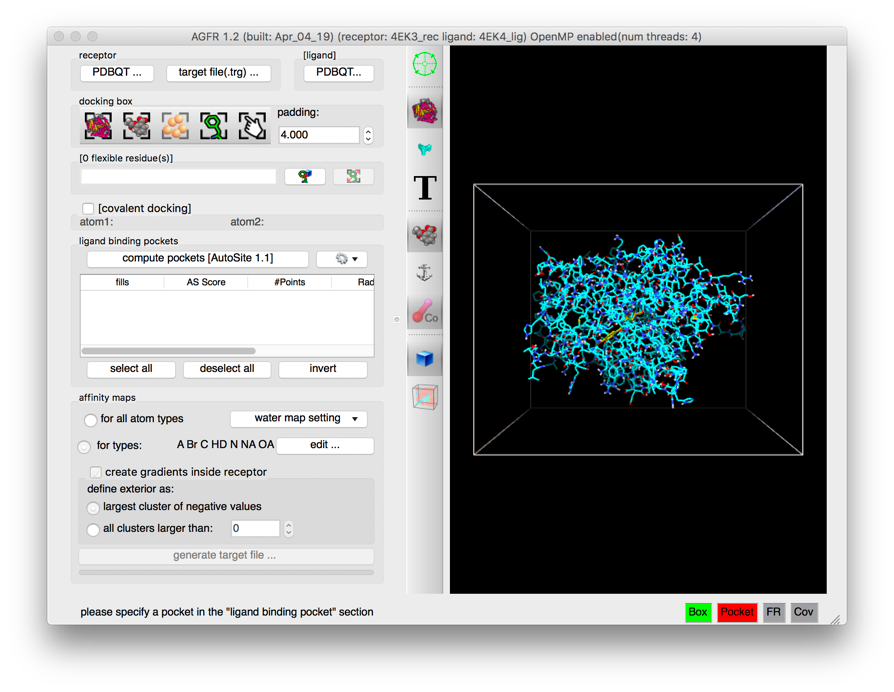
NOTE:
- More buttons are enabled by this operation both in the control panel and in the tool-bar. In particular the button allowing to position and size the box using the ligand
 .
.
Click  to make the docking box cover the ligand then click on
to make the docking box cover the ligand then click on  to focus the 3D scene on the box.
to focus the 3D scene on the box.
Details: the docking box is now centered on the ligand and scaled to have a 4.0 angstroms padding from the ligand atoms.
The padding can be adjusted using the spin box in the “docking box” section.
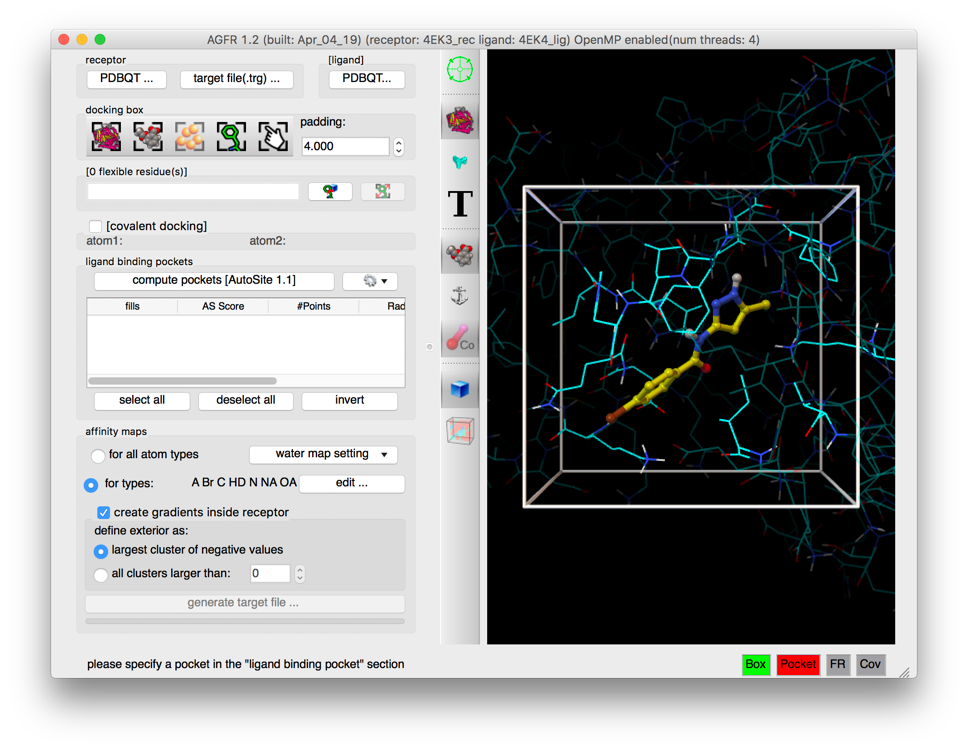
NOTES:
- The amino acids outside the docking , as well as the amino acids within the docking box with no flexible side-chains (i.e. glycine, alanine and proline) are displayed dimmed down. This facilitates the visual identification of the amino acids in the docking box that can potentially be made flexible during the docking calculation.
- Click the
 button to display the “box parameters” user interface. This interface provides widgets to modify the box center coordinates, and box size along the 3 dimensions.
button to display the “box parameters” user interface. This interface provides widgets to modify the box center coordinates, and box size along the 3 dimensions.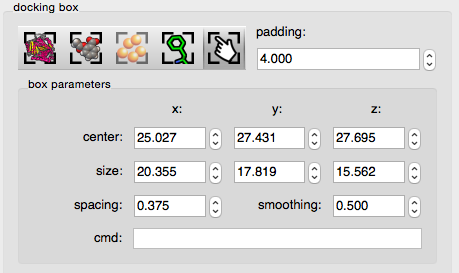 NOTE: the mouse scroll wheel can be used to alter values while the mouse pointer is over the widget displaying numerical values.
NOTE: the mouse scroll wheel can be used to alter values while the mouse pointer is over the widget displaying numerical values.
The spacing widget sets the distance between the grid points at which affinity values are calculated.
The smoothing widget allows the specification of the smoothing factor used for calculating affinity values. These values are initialized with AutoGrid’s default values.
The cmd typein widget allows the command-line specification of the docking box. For instance: center 10 11 12.3 will center the docking-box on (10.0, 11.0, 12.3). Multiple commands can be entered on a single line separated by a semicolon character ‘;’ (e.g. “center 12.34 34.12 56.56; size 26 26 26″) .
Click on  and select 1.0
and select 1.0
Details: versions 1.0 and 1.1 of AutoSite are available. Version 1.1 produces better pocket descriptions, especially for larger ligands, but is somewhat slower. Here we use version 1.0 as it displays more grid points which work better for illustrative purposes.
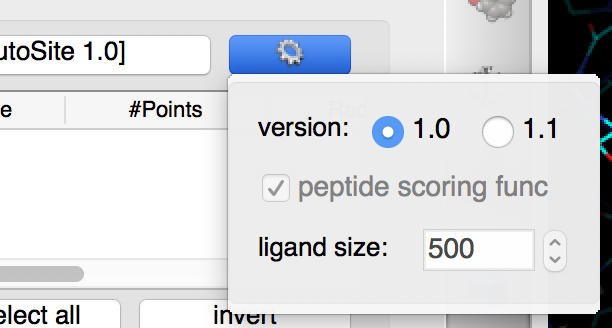
NOTES:
- Version 1.1 of AutoSite performs post processing steps on the fills identified by version 1.0. First it inflates the fills to achieve better coverage of known ligands, and second it shrinks the inflated fill to retain 1/5 of the fill points to generate better points to be used during docking to position the ligand in the box.
- Version 1.1 can use a custom scoring function for ranking pockets when the ligand is a peptide.
- Version 1.1 uses a ligand size (i.e. volume expressed in grid points) to rank pockets. The default values of 500 selects good pockets for both small molecules and peptides.
Click on 
Details: ligand binding pockets are computed using AutoSite by identifying and cluster high affinity points. Each cluster of points is called a “fill” and listed in the table widget. The pocket with the largest AutoSite score is selected by defaults and displayed as a set of green dots. The fill points of all selected fills are displayed in the 3D viewer and will be written into the target files.
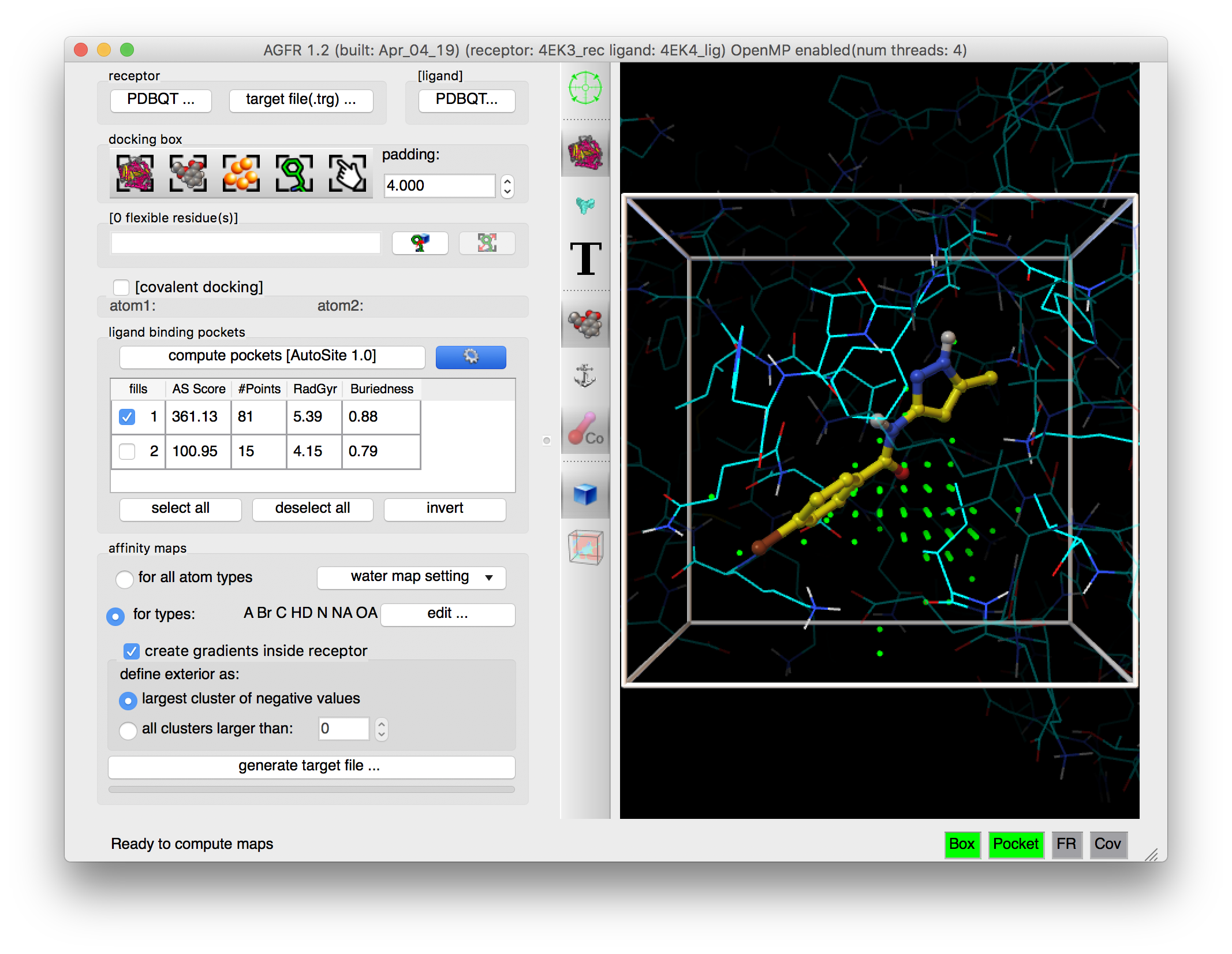
NOTES:
- AGFR supports version 1.0 and 1.1 of the AutoSite algorithm. The latter is slower but creates better pocket descriptors, in particular for larger ligands such as peptides.
- Binding pockets are defined as subsets of high affinity grid points calculated on a 1 Angstrom resolution grid for the carbon, oxygen, and hydrogen maps (C, NA, HD). These points are clustered. Each cluster is called a fill and is assigned a rank. Usually, high ranking clusters are likely to be ligand binding pockets.
- Additional fills can be selected. The fill points of all selected fills are displayed as dots and will be saved in the target file. ADFR will use these points during docking for positioning the ligand in the docking box.
- The button labeled “generate target file…” is now enabled and all required status lights are green indicating that we defined a valid docking box and a valid pocket definition (i.e. a set of fill points that overlap with the docking box).
Check the  button
button
Details: loading the ligand initialized the list of maps to be computed to the list of AutoDock atom types found in the ligand, in this case: “A C Cl HD N NA OA”. Computing maps for all atoms types will use a little more disk space but the resulting target file can be used for any ligand and is recommended.
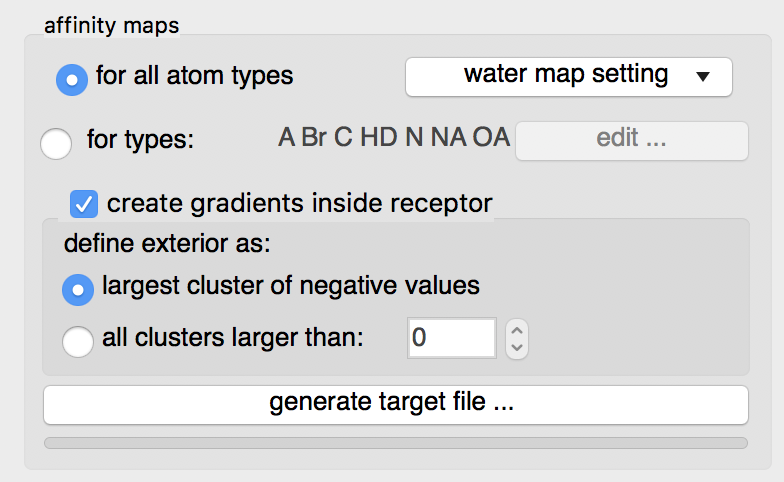
The “edit …” button will display an interface for manually specifying the list of atoms types for which affinity maps are requested.
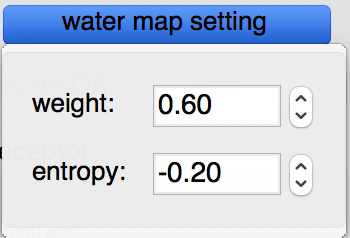
Water maps: water maps are calculated automatically and stored in the target file. These maps allow to perform hydrated docking. the parameter for these maps can be set in the “water map setting” pull down.
Gradients: By default, an affinity gradient will be created for the region of the grid covered by the receptor (except for the electrostatic and desolvation maps). If your CPU has multiple cores and OpenMP is detected, the calculation will execute in parallel and the number of available threads appears in the GUI title bar. Gradients are not required, however, they are recommended as they have been shown to help docking find solutions more efficiently. The addition of gradients requires the definition of the interior and exterior of the receptor. By default, the largest cluster of negative values (favorable affinities) is used to define the exterior of the receptor. In this case, everything else, including receptor cavities large enough to hold solvent but not open to the solvent, will be considered the interior and will be overwritten with the gradient. To prevent the gradient generation on grid points of internal cavities, the user can specify a cluster size above which clusters of points with negative values are considered to be outside the receptor and therefore preserve their original affinity values.
Click on the “generate target file…” button
Details: the program asks for the name and location for the target file that will be generated. It will then compute the affinity maps and store them in the target file along with meta data.
The generated target file describes the binding pocket targeted for docking on the receptor. It stores the PDBQT file of the receptor used to compute the affinity maps, the grid parameter file used to run AutoGrid, as well as files generated by the AutoGrid run (i.e. affinity maps, AutoGrid log file, etc.). In addition, it stores meta-data about the generation of the target file such as: the time and computer architecture on which the maps were computed, the docking-box parameters, the grid parameter file, the versions of AGFR and AutoSite, the water map parameters, etc.
It is recommended to use names that are descriptive. In this case we use NativeCDK2BindingSite_rigid as we defined the docking box using the native ligand and we did not specify any flexible receptor side chains.
Pressing the “Save” button will start the calculation in a separate thread, leaving the graphical user interface responsive. The progress bar below the button will indicate the level of completion of the calculation.
NOTE: a target file can be inspected from the command line using the about command (AutoDockSuite-1.0/bin/about)
Copy to Clipboard
Details: The target file produced by this tutorial can be generated from the command line using this command.
-r : specifies the receptor file
-l : specifies the ligand file
-as : tells AGFR to use the original AutoSite algorithm rather than version 1.1
NOTE: By default, when a ligand is specified on the command line, AGFR will use this ligand to position and size the box. For more command line options use the -h/–help command line argument with the agfr command.
The command provided above produces the following output: